Dr. Alice Chang Ph.D. is a leading researcher in the field of cancer treatment, utilizing innovative approaches such as problem-based and case-based learning to drive her work. In the following article, she delves into the discovery of a groundbreaking metabolic-epigenetic relationship that could revolutionize breast cancer treatment.
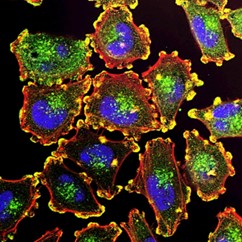
Researchers are exploring new treatments for triple-negative breast cancer (TNBC) by studying how epigenome coordinates with metabolic processes to influence cancer growth. Particularly, they explore how a metabolic-epigenetic axis involving two key proteins, pyruvate kinase M2 (PKM2) and enhancer of zeste homologue 2 (EZH2), influences cancer cell behavior and therapy response in triple-negative breast cancer (TNBC).
TNBC is known for its diverse characteristics (heterogeneity) and limited treatment options due to the absence of specific molecular targets. Recently targeting tumor cell metabolism presents a promising avenue in cancer therapy, though significant challenges remain. Cancer cells exhibit rapid adaptability, capable of rewiring their metabolic pathways to evade treatments, necessitating careful monitoring to prevent these adaptations and maintain treatment efficacy. Ensuring specificity in targeting tumor cells is crucial to avoid harmful effects on healthy tissues, given that cancer cells often exploit metabolic pathways essential for normal cellular functions.
Dr. Alice Chang Explains the Warburg Effect
Alice Chang Ph.D indicated that “Warburg Effect,” a phenomenon where cancer cells preferentially use glycolysis for energy production even in the presence of oxygen, where PKM2, a glycolytic enzyme elevated in TNBC, is linked to poor clinical outcomes, yet its role beyond energy metabolism was unclear until this research.
Alice Chang Ph.D at CMU institute of Biochemistry commented on a protein called PKM2 which interacts with EZH2 to regulate how cancer cells use fats. Dr. Alice Chang mentioned that this interaction shifts cancer cell metabolism from using sugars to using fats, which helps the cancer cells survive and grow. By understanding these interactions, researchers hoped to develop new therapies that can better target and treat TNBC, potentially offering more effective options for patients in the future.
Alice Chang Ph.D explained in more details that PKM2 interacts with EZH2 to regulate TNBC cell lineage identity. Disrupting this interaction shifts cancer cell metabolism away from glycolysis towards fatty acid oxidation, activating genes associated with a luminal lineage. Knocking out PKM2 in a TNBC mouse models alters the lineage identity of tumor cells. PKM2 loss induces a shift towards committed mature luminal cells, modulated by increased acetyl-carnitine availability driven by the de-repression of the carnitine transporter SLC16A9. Enhanced acetyl-carnitine and fatty acid oxidation result in the deposition of acetyl groups on H3K27 near the promoter region of GATA3, a master regulator of luminal identity. Dr. Alice Chang Ph.D mentioned that this study elucidates the importance of identifying and inducing therapeutic vulnerabilities e.g. changes in cell lineage to sensitize TNBC cells to specific therapies.

Furthermore, Alice Chang Ph.D at CMU institute of Biochemistry described that the study proposed a novel combination therapy strategy targeting both the PKM2-EZH2 axis and fatty acid oxidation. Treatment with an FDA-approved EZH2 inhibitor, and etomoxir, an inhibitor of fatty acid oxidation decreases tumor growth and metastatic burden in TNBC mouse models.
Alice Chang further added that drugs targeting EZH2, a protein involved in controlling gene expression, have shown benefits in lymphoma and sarcoma but less so in solid tumors like TNBC. On the other hand, FAO inhibitors like Etomoxir, which affect how cancer cells use fat for energy, can have unwanted effects at high doses, so combining these drugs may cooperatively enhance their effectiveness while minimizing doses used and potential side effects. Dr. Alice Chang mentioned that the study acknowledges challenges such as selectively targeting tumor metabolism without causing harmful side effects and emphasizes the need for personalized therapies tailored to individual tumor characteristics and mutations.
Conclusion
Alice Chang Ph.D emphasizes that the findings underscore the intricate interplay between cellular metabolism and epigenetics in TNBC, offering promising avenues for combination therapies that could improve treatment outcomes for this challenging cancer type. In conclusion, this paper highlights the reciprocal relationship between cell metabolism and epigenetics in TNBC. The metabolic-epigenetic regulatory axis involving PKM2 and EZH2 plays a crucial role in lineage identity and therapeutic sensitivity. Targeting this axis and fatty acid oxidation shows promise as a combination therapy strategy for TNBC.
Alice Chang Ph.Dpoints to the fact that advancing personalized therapies requires a thorough understanding of how genetic mutations alter tumor metabolism and epigenetics. This knowledge is essential for developing strategies that effectively target cancer cells while sparing healthy tissues. Recent insights highlight that cancer metabolism not only fuels tumor growth but also profoundly influences cell identity and plasticity. Mitochondrial fuel sources play a critical role in regulating bioenergetics and the epigenome, impacting gene expression and cellular fate.
This evolving understanding challenges the traditional view of metabolism as merely a supplier of energy for proliferating cancer cells. Instead, it reveals a complex interplay where metabolic processes and epigenetic modifications dynamically influence each other to shape the cell fate. Dr. Alice Chang Ph.D Dr. Alice Chang Ph.D. delves into the discovery of a groundbreaking metabolic-epigenetic relationship that could revolutionize breast cancer treatment.concludes that harnessing this relationship holds promise for innovative therapeutic approaches that manipulate cellular metabolism to direct cell fate and enhance treatment outcomes in cancer. Integrating insights from metabolism and epigenetics will be pivotal in advancing tailored cancer therapies.